Introduction: Ibrutinib (I), an irreversible Btk inhibitor, and venetoclax (V), a Bcl-2 inhibitor, improve CLL outcomes in trials compared to chemoimmunotherapy. I and V target two key pathophysiological pathways in CLL and should be synergistic. This is supported both by in vitro studies and Phase II trials in which I+V results in high proportions of measurable residual disease (MRD) negativity. A Phase III trial comparing I+V (15 months [mo]) with chlorambucil-obinutuzumab led to the approval of I+V. However, mathematical disease modelling and Phase II studies favor defining duration of I+V according to individual patient sensitivity. We hypothesized that I+V is more effective than FCR in CLL and that treatment duration personalised using MRD response would optimize outcome.
Methods: FLAIR (ISRCTN01844152) is a phase III, multicentre, randomised, controlled, open, parallel group trial for untreated CLL. Patients (pts) with >20% 17p deleted cells were excluded. FLAIR was adapted in 2017 to add 2 arms, I alone and I+V compared to FCR. Here we report the planned analysis of I+V vs FCR. In I+V after 2 mo I, V was added with a 4-week dose escalation to 400mg/day and then I+V for up to 6 years with duration of I+V defined by MRD (<1 CLL cell in 10,000 [flow cytometry]). PB MRD was assessed at 12 mo and then 6 monthly and if negative, was repeated at 3 mo and 6 mo in PB and BM. If all were MRD neg, then the duration of I+V was double the time between start of I+V and the initial MRD neg PB (I+V duration: 2 to 6 years). The primary endpoint for I+V vs FCR was investigator-assessed PFS. Key secondary endpoints presented were OS, IWCLL response, MRD and safety. Appropriate endpoints were analysed by CLL prognostic sub-groups.
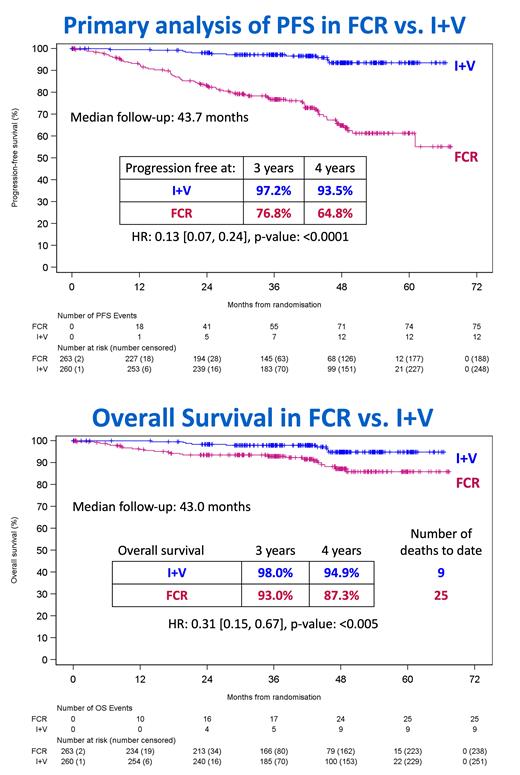
Results: 523 pts were randomised to FCR (n=263) and I+V (n=260) at 96 UK Centers from 07/20/2017 to 03/24/2021. Data-lock on 05/23/2023. 71.3% male, median age 62 yrs (31.2% >65yo) and 40.9 % Binet Stage C. IGHV unmutated (≥98% homology to germline) in 56.9%, 37.6% IGHV mutated and 5.5% Subset 2. Hierarchical FISH: 20.6% 11q del, 20.1% trisomy 12, 27.8% normal and 31.4% 13q del. At 2 yrs 111/260 (42.7%) and 3 yrs 135/232 (58.1%) pts stopped I+V according to the MRD stopping rules. At a median 43.7 months there were 87 progressions - 75 FCR and 12 I+V. The hazard ratio (HR) for PFS for I+V vs FCR is 0.13 (95% CI: [0.07, 0.24]; p<0.0001; Fig). This result was consistent for gender, age or stage. At 3 yrs 2.8% had progressed on I+V compared to 23.2% on FCR. There have been 34 deaths (25 FCR and 9 I+V) resulting in improved overall survival for I+V vs FCR: HR 0.31 (95% CI: [0.15, 0.67]; p=0.0029; Fig). At 3 years 2.0% of I+V pts had died compared to 7.0% for FCR. At 9 months (3 mo post-FCR) 48.3% FCR pts became MRD neg in BM compared to 41.5% for I+V. However, with continued I+V more pts became MRD neg: the odds of MRD negativity at any time for I+V vs FCR were 2.03 (95% CI: [1.43, 2.89]; P<0.001) in BM and 3.91 (95% CI: [2.55, 6.00]; P<0.001) in PB. 90.6% pts achieved PB MRD negativity at up to 5 yrs I+V and 88% of these were BM MRD negative 6 mo after their first PB MRD neg result. At 9 months a higher proportion achieved CR and overall response for I+V; CR - FCR 49.0% (95% CI: [42.9%, 55.3%]), I+V 59.2% (53%, 65.3%); ORR - FCR 76.4% (70.8%, 81.4%); I+V 86.5% (81.8%, 90.4%). This difference was greater for best response at any time: ORR 83.7% (78.6%, 87.9%) for FCR vs 95.4% (92.1%, 97.6%) for I+V; CR 71.5% (65.6%, 76.9%) for FCR vs 92.3% (88.4%, 95.2%) for I+V. The odds ratios estimate to achieve CR with I+V vs FCR is 1.51 (95% CI: [1.07, 2.14]; p<0.05). Responses and outcomes by FISH and IGHV will be presented. SAEs were reported in 252 (51.3%) pts (129 FCR vs 123 I+V). Notable SAEs by organ class for FCR vs I+V were: infections 18.8% of FCR pts vs 22.2% for I+V; blood and lymphatic 31% vs 5%; and cardiac in 0.4% vs 10.7%. 4 pts had sudden or cardiac deaths - 2 FCR and 2 I+V. 69 other cancers were diagnosed (45 in FCR, 24 in I+V) in 51 pts (34 FCR, 17 I+V). The incidence of other cancers per 100 pt-years was greater for FCR than I+V; 5.4 (95% CI: [5.11, 5.68]) vs. 2.6 (2.40, 2.79). There were 7 cases of MDS/AML with FCR and 1 with I+V.
Conclusion: Ibrutinib plus venetoclax significantly improved progression-free and overall survival compared to FCR in untreated CLL. Using MRD to direct the duration of I+V maximizes outcome with 97.2% progression free survival at 3 years The efficacy seen in FLAIR is superior to previous Phase III CLL trials indicating that I+V with duration guided by MRD is a new gold standard for CLL treatment.
Disclosures
Hillmen:Apellis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Cairns:Janssen: Honoraria; Celgene BMS: Honoraria, Research Funding; Sanofi: Research Funding; Amgen: Research Funding; Takeda: Research Funding. Bloor:Abbvie: Consultancy, Honoraria, Speakers Bureau; Gilead, Janssen: Honoraria. Cwynarski:Abbvie: Membership on an entity's Board of Directors or advisory committees; : Roche, Takeda, Celgene, Atara, Gilead, KITE, Janssen, Incyte, Abbvie: Consultancy, Honoraria; Roche, Takeda, KITE, Gilead, Incyte: Speakers Bureau; Roche, Takeda, KITE, Janssen, BMS: Other: Conferences/Travel support. Paneesha:Gilead: Honoraria; Abbvie: Honoraria; Janssen: Honoraria; Astra Zeneca: Honoraria. Fox:AbbVie: Consultancy; Genmab: Consultancy, Membership on an entity's Board of Directors or advisory committees. Eyre:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Loxo Lilly: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medscape: Speakers Bureau; PeerView: Speakers Bureau; KITE: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Autolus: Consultancy; Eli Lilly and Company: Consultancy, Honoraria, Speakers Bureau; Loxo Oncology: Consultancy, Honoraria, Other, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees. Gribben:AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Speakers Bureau; Kite, A Gilead Company: Consultancy, Speakers Bureau; Janssen Pharmaceuticals, Inc: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy; Bristol Myers Squibb: Speakers Bureau. Howard:Roche: Current Employment. Hockaday:Abbvie: Speakers Bureau. Rawstron:Abbvie, BD Biosciences, Beckman Coulter, Beigene, Celgene, Gilead, Janssen, Pharmacyclics, Roche: Research Funding; Beigene, Pharmacyclics: Consultancy; BD Biosciences: Patents & Royalties; Abbvie, Beigene, Innocare, Janssen, Medicxi, Thermo Fisher: Honoraria. Patten:Roche: Honoraria, Research Funding; Novartis: Honoraria; AbbVie: Honoraria, Other: Meeting Support; Astra Zeneca: Honoraria; Beigene: Honoraria, Other: Meeting Support; Kite / Gilead: Honoraria, Research Funding; Janssen: Honoraria, Other: Meeting Support. Munir:BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal